Surface Science Group


SUGIMOTO GROUP

-
Associate ProfessorToshiki SUGIMOTO
-
Assistant ProfessorAtsunori SAKURAI
RESEARCH

Unveiling exotic structural properties, chemical functions
& quantum dynamics of interfacial water molecules
Interfacial phenomena, in which water molecules are deeply involved, are critical subjects in basic and applied research in a wide range of fields, including physics, chemistry, mechanical engineering, biology, geology, and astronomy.
We lead basic research in unveiling these academically and practically fascinating phenomena of water at atomic/molecular levels. We have tackled the challenge of building human knowledge and basic theory that are of fundamental importance for innovative development toward the 22nd century.
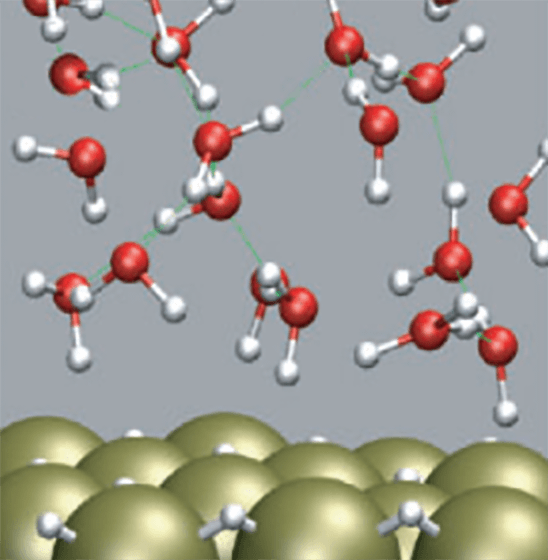
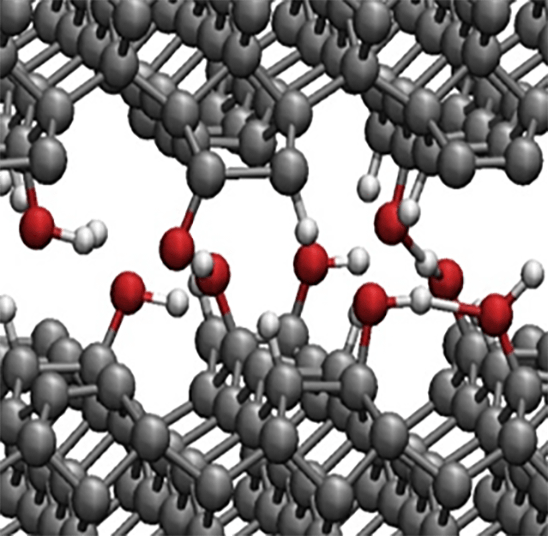
Water molecules are one of the most familiar and abundant molecules in nature, and they typically aggregate (adsorb) on the surfaces and interfaces of various substances. Under such circumstances, water molecules behave quite differently from those in gas and liquid phases, and an unexpected molecular process sometimes occurs. There are many important applications where surfaces/interfaces play crucial roles: photocatalytic reactions, friction, adhesion, chemical and physical processes in interstellar/stratospheric environments, electrification in thunderclouds, and biological phenomena woven by DNA, protein, and cells, etc. To elucidate molecular-level characters and functions of water molecules in these heterogeneous processes, a basic understanding is necessary about the peculiar hydrogen-bond structures of the interfacial water systems. In these symmetry-breaking systems, adsorption geometry and molecular orientation are key structural parameters that determine the functional properties of interfacial water molecules.
-
Electrochemistry
Catalysis etc. -
Tribology
Adhesion etc. -
Astrochemistry
Astrophysics etc. -
Biology
Physiology etc
In the case of water, molecular orientation is determined by the position of hydrogen. Since hydrogen is the lightest element with the smallest number of electrons attached, conventional experimental techniques used as structure analysis methods are mostly insensitive to the position of a small number of hydrogens (~1014cm-2) in the interfacial hydrogen-bond network. Therefore, accessing the microscopic information on the orientation of interfacial water molecules (H-up/H-down arrangement of hydrogen) has been a long-standing experimental challenge.
To unveil the configuration of hydrogen (H-up/H-down orientational arrangement) in a water molecular system, we have focused on leading-edge laser-based molecular spectroscopy such as second-order nonlinear optical sum-frequency generation (SFG) spectroscopy. With this advanced method, we have challenged elucidating the mechanism that hydrogen-bonded systems of water molecules exert unique structural properties, chemical functions, and quantum dynamics by focusing on the surface/interface of practical materials as well as model systems with well-defined and controlled nanostructures.
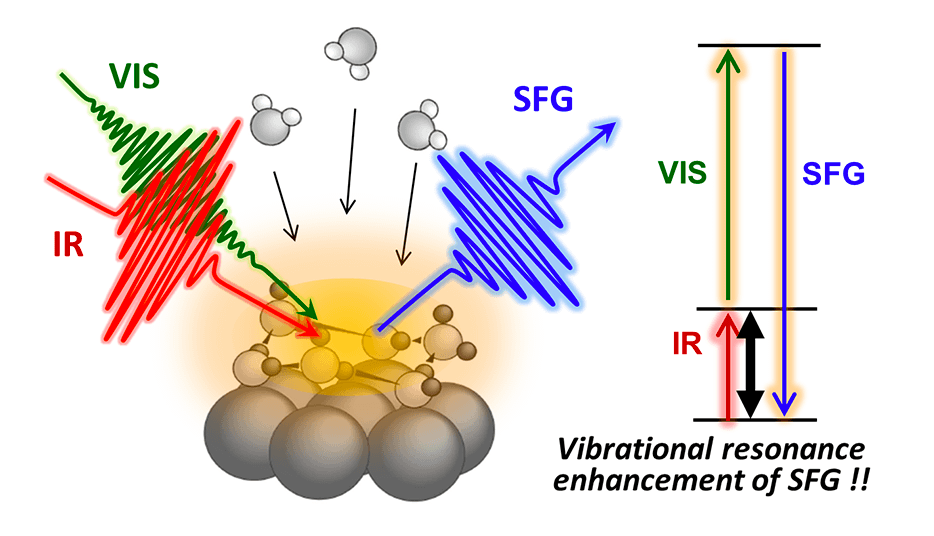 sum-frequency generation (SFG) vibrational spectroscopy with simultaneous irradiation of infrared and visible laser pulse
sum-frequency generation (SFG) vibrational spectroscopy with simultaneous irradiation of infrared and visible laser pulse
When the frequency of incident infrared light resonates with the characteristic frequency of molecular vibrational mode, light with the sum frequency of infrared light and visible light is generated by a second-order nonlinear optical effect. State-of-the-art observation of this sum-frequency light with "heterodyne detection" method enables us to determine the H-up/H-down configuration of hydrogen in water molecular systems.
Our group has succeeded in applying this observation method to water molecular systems on solid surfaces for the first time [Nature Phys., (2016)] and has been engaging in pioneering research in the field.
We also have made great efforts to develop cutting-edge experimental methods by combining various light (continuous light / pulse laser light) from infrared to X-ray region, electron beam, and scanning probe microscope (SPM). Our new experimental approaches open up novel routes to provide genuinely beneficial and essential molecular-level insights into surface/interfacial phenomena in which water molecules and hydrogen are deeply involved.
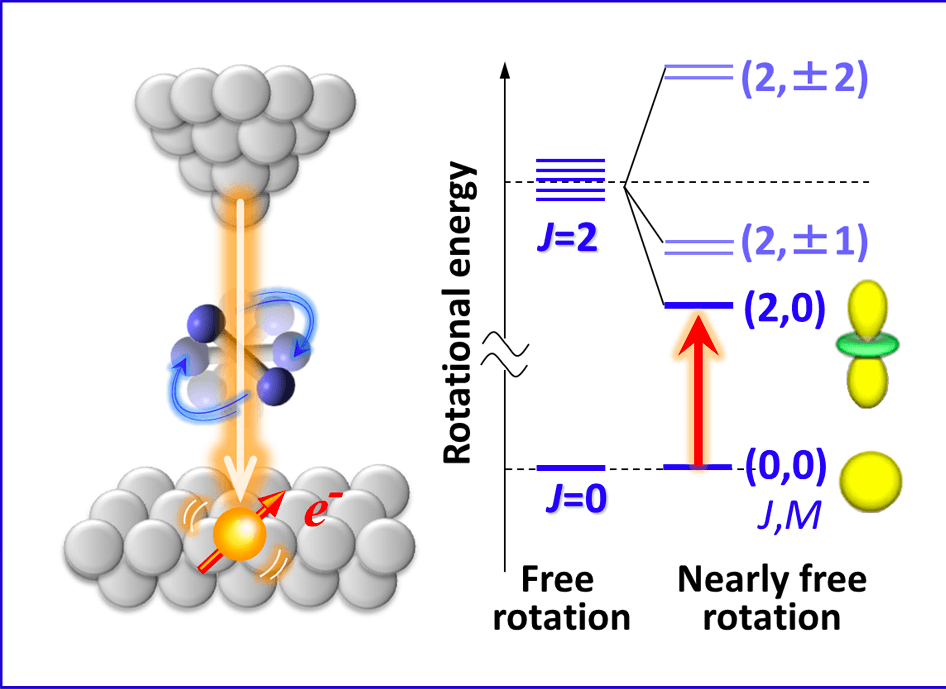 single-molecule level microspectroscopy for observing the structure and rotational and vibrational motion of molecular aggregates on solid surfaces
single-molecule level microspectroscopy for observing the structure and rotational and vibrational motion of molecular aggregates on solid surfaces
It has been tough to theoretically describe the inelastic scattering process of tunneling electrons via rotational excitation of molecules. For the first time, we have succeeded in constructing an appropriate theoretical framework to work with this process based on the second-order level perturbation theory and reporting the underlying physical mechanism by explicitly taking into consideration quantum angular momentum [Phys. Rev. B (Rapid communication) , (2017)]. By applying this method, we have made great efforts to develop more sophisticated and innovative experimental methods that can microscopically elucidate unique hydrogen-bond structures and dynamism that stimulate new physical properties and chemical functions of interfacial water systems.
We take the initiative in basic research to advance innovation in controlling specific hydrogen bonds of water molecules (hydrogen local structures) at surfaces/interfaces. Microscopic and essential knowledge provided in our group will contribute to designing and stimulating valuable emergent physical/chemical properties and biological functions.
There are other research topics not listed here that are currently in progress. Contact us if you are interested in basic research in our group.

RECRUIT


Opportunities for Professionals
Postdocs / Research Assistant Professors / Technical Fellows
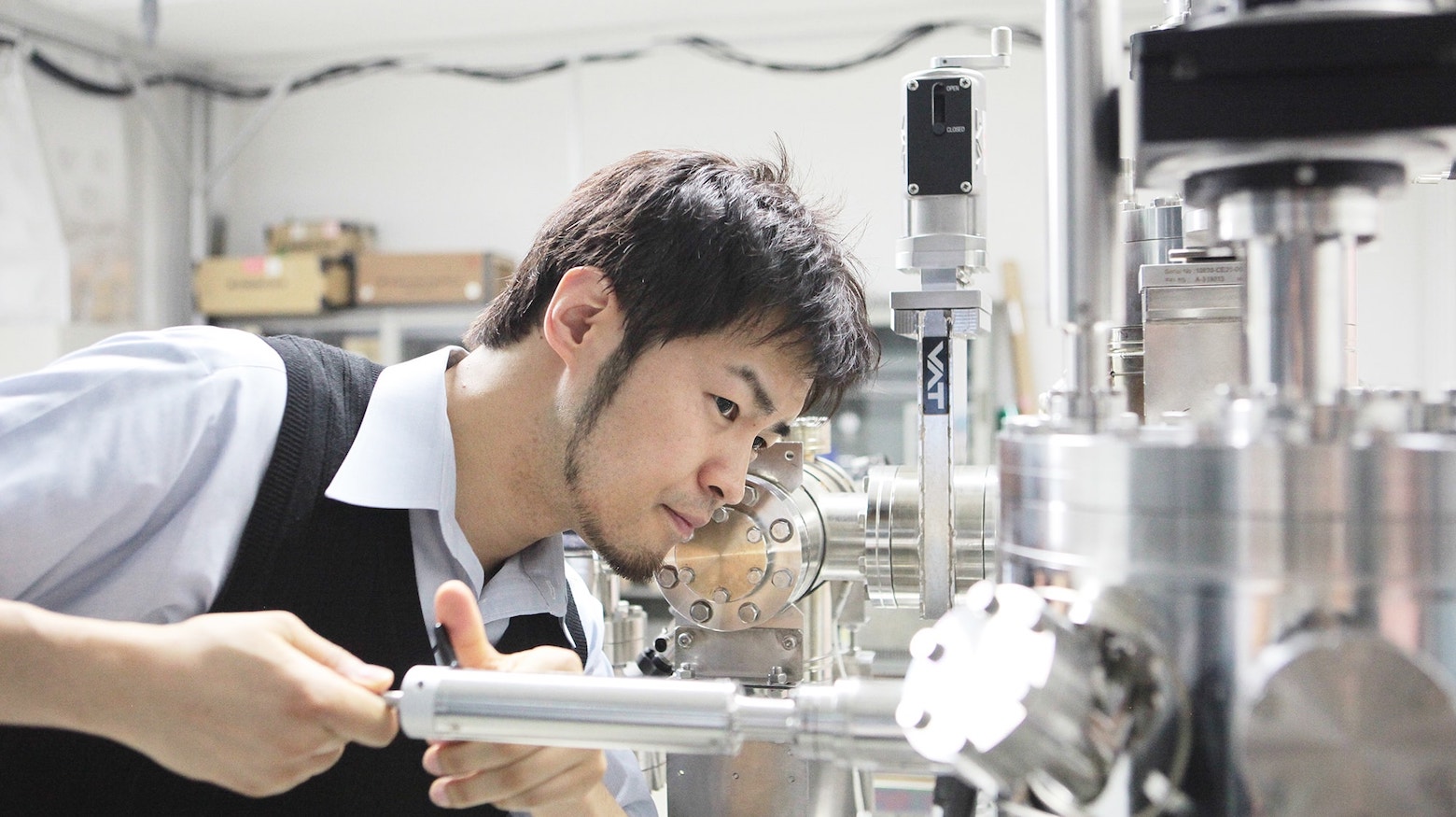
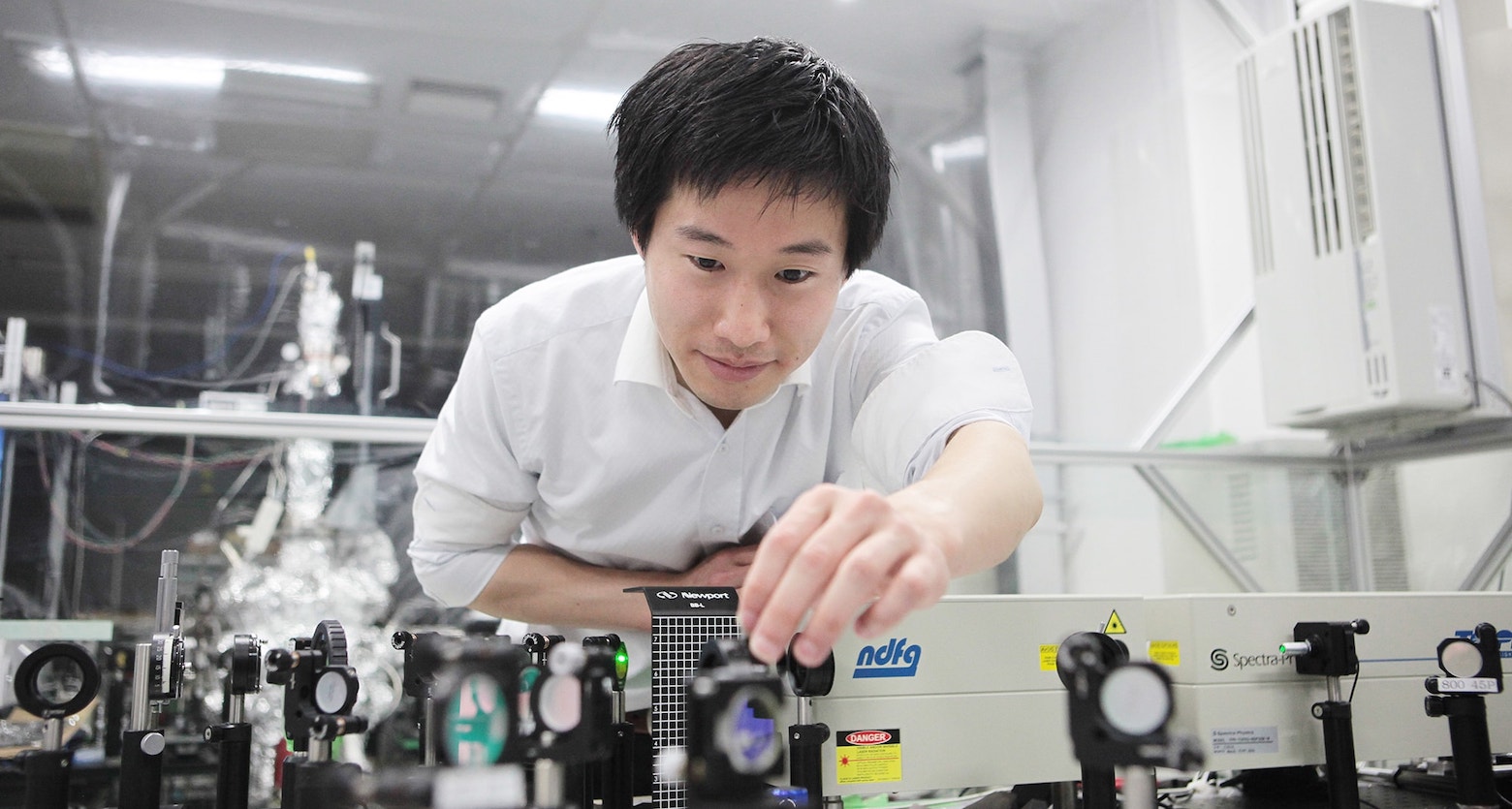
We develop and leverage novel nanoscale spectroscopy to probe the fundamental structure and dynamics of water molecules at surfaces and interfaces. We are currently seeking highly motivated and skilled young researchers to join our collaborative, interdisciplinary group.
You will be responsible for state-of-the-art non-linear optical spectroscopy and molecular scale nanoscopy to investigate water orientation in-situ or in operando concerning specific surface sites.These experimental approaches are the first crucial step toward understanding designing and controlling the unique properties of interfacial water systems!

Interested applicants should submit the following materials to us at bunshiken-sugimotogroup@ims.ac.jp.
1. A letter of interest (max. 1 page), clearly stating your specific interest to join the group.
2. CV (Previous experience with laser-based spectroscopy, or surface science is an advantage.)
You can also join us as a JSPS research fellow.
For inquiries, please contact Toshiki Sugimoto.

